 |
 |
All products are Prescription Strength
Shake Powder
Liquid Spray
Spray Powder
Jock Itch Spray Powder
Indications: Cures athlete's foot (tinea pedis), jock itch (tinea cruris) and ringworm (tinea corporis). For effective relief of the itching, burning, cracking and scaling which can accompany these conditions. Desenex powders also help keep feet dry.
Active Ingredient: Shake Powder , Liquid Spray , Spray Powder , and Jock Itch Spray Powder --Miconazole nitrate 2%.
SHAKE POWDER --Corn starch, corn starch/acrylamide/sodium acrylate polymer, fragrance, talc.
LIQUID SPRAY --Polyethylene glycol 300, polysorbate 20, SD alcohol 40-B (15% w/w).
Propellant: Dimethyl ether.
SPRAY POWDER --Aloe vera gel, aluminum starch octenyl succinate, isopropyl myristate, propylene carbonate, SD alcohol 40-B (10% w/w), sorbitan monooleate, stearalkonium hectorite.
Propellant: Isobutane/propane.
JOCK ITCH SPRAY POWDER --Aloe vera gel, aluminum starch octenyl succinate, isopropyl myristate, propylene carbonate, SD alcohol 40-B (10% w/w), sorbitan monooleate, stearalkonium hectorite.
Propellant: Isobutane/propane.
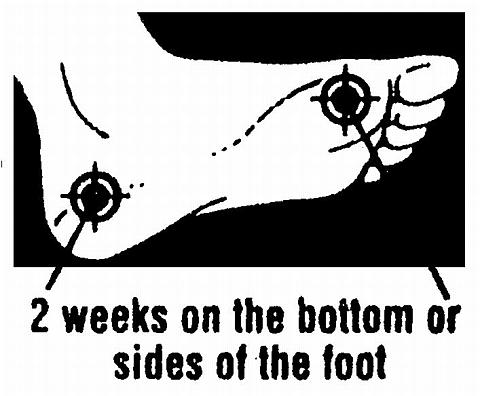
Warnings: Do not use on children under 2 years of age unless directed by a doctor. For external use only. Avoid contact with the eyes. If irritation occurs, or if there is no improvement within 4 weeks (for athlete's foot or ringworm) or within 2 weeks for jock itch, discontinue use and consult a doctor. Keep this and all drugs out of the reach of children. In case of accidental ingestion, seek professional assistance or contact a poison control center immediately. Use only as directed. For Spray Powders and Liquid Spray --Avoid inhaling. Avoid contact with the eyes or other mucous membranes. Contents under pressure. Do not puncture or incinerate. Flammable mixture, do not use near fire or flame. Do not expose to heat or temperatures above 49°C (120°F). Use only as directed. Intentional misuse by deliberately concentrating and inhaling the contents can be harmful or fatal.
Directions: Clean the affected area and dry thoroughly. Apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor. (For Sprays: Shake Spray can well, and hold 4[Prime ] to 6[Prime ] from skin when applying.) For athlete's foot, pay special attention to the spaces between the toes. Wear well-fitting, ventilated shoes and change shoes and socks at least once daily. For athlete's foot or ringworm, use daily for 4 weeks. For jock itch, use daily for 2 weeks. If condition persists longer, consult a doctor. Supervise children in the use of this product. This product is not effective on the scalp or nails.
Shake Powder --1.5 oz, 3 oz, 4 oz. plastic bottles.
Spray Powder --3 oz, 4 oz. cans.
Liquid Spray --3.5 oz, 4.6 oz. cans.
Store powders/spray powders and liquid sprays at room temperature, 15°-30°C (59°-86°F). See container bottom for lot number and expiration date. Spray powders: Tamper-resistant aerosol can for your protection. If clogging occurs, remove button and clean nozzle with pin.
DesenexMax Cream®
Active Ingredient: Terbinafine hydrohloride 1%
Inactive Ingredients: benzyl alcohol, cetyl alcohol, cetyl palmitate, isopropyl myristate, polysorbate 60, purified water, sodium hydroxide, sorbitan monostearate, stearyl alcohol.
Warnings: For external use only. Do not use:
When using this produt do not get into the eyes. If eye contact occurs, rinse thoroughly with water. Stop use and ask a doctor if too much irritaiton occurs or gets worse. Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
Directions: Adults and children 12 years and over:
 |
 |
How Supplied: 0.42 oz. (12 gram) and 0.85 oz. (24 gram) tubes
Do not use if seal on tube is broken or is not visible. Store between 5° and 30° C (41° and 86° F).
Questions: Call 1-800-452-0051, 24 hours a day, 7 days a week
Novartis Consumer Health Inc.
Summit NJ 07901-1312
©2001
 |